Amino Acid
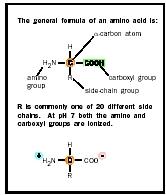
Amino acids are molecules that have both an amino group (-NH 2 ) and a carboxylic acid group (-COOH), hence the name. The most common amino acids are the α-amino acids, the building blocks of proteins . These have the amino group, the carboxylic acid group, a hydrogen, and a characteristic side chain all attached to one carbon atom, designated the α-carbon. Each type of α-amino acid has a unique side chain that determines its properties and its role in proteins. The side chains (or "R" groups) can range from a hydrogen atom, as in glycine, to the more complicated side chains of tryptophan or arginine.
The α-carbon atom has four different groups attached to it arranged at the points of a tetrahedron. This arrangement is asymmetric and can occur in two different forms, or enantiomers, that are related to each other in the same way as an object and its image in a mirror. These two enantiomers are called L and D. Only L-amino acids occur in proteins made by living systems. D-amino acids and amino acids other than α-amino acids occur in biological systems but are not incorporated into proteins.
Many organisms can synthesize all of the amino acids they require from compounds present in the metabolic pathways they use for energy production. Humans, however, are not able to synthesize all of the necessary amino acids, and a number of them must be obtained from the diet.
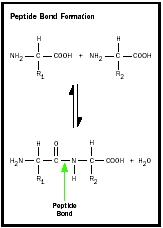
The major use of amino acids is to construct proteins. A protein is a linear chain of amino acids linked together by peptide bonds . A peptide bond is formed when the amino group attached to the α-carbon of one amino acid is joined to the carboxyl group of a second amino acid with the elimination of water. The side chain of each amino acid residue protrudes from the polypeptide backbone. The sequence of amino acids in the chain is determined by the deoxyribonucleic acid (DNA) sequence of the gene that codes for that protein.
The three-dimensional structure and the properties of a specific protein, and therefore its biological role, are determined by the sequence of
A protein folds so that nonpolar side chains tend to be buried within the protein while polar and charged side chains tend to be exposed to the water around the protein. The biological function of a protein is generally highly dependent on its three-dimensional structure.
SEE ALSO Enzymes ; Protein Structure
Wayne F. Anderson
Bibliography
Alberts, Bruce, et al. Molecular Biology of the Cell, 4th ed. New York: Garland Publishing, 2000.
Stryer, Lubert. Biochemistry, 4th ed. New York: W. H. Freeman and Company, 1995.
Comment about this article, ask questions, or add new information about this topic: