Herbal Medicine
Using medicines derived from plants is a practice probably as old as humankind itself. Prehistoric peoples likely noted when consuming a particular plant part provided relief, such as willow bark "tea" lowering a fever. Sumatran clay tablets engraved forty centuries ago list plant-based remedies for common ills, as do ancient writings from Egypt and China. In nineteenth-century United States, St. John's wort and Echinacea were just two of many commonly used herbal remedies.
Many modern medicines are synthetic versions of plant-derived "natural products." A compound from a periwinkle plant, for example, served as the basis for a powerful drug that fights leukemia. Poppies provide alkaloids such as morphine that are potent painkillers.
In the U.S. today, one-third of all adults have tried herbal treatments, creating a multibillion-dollar market. The resurgence of interest in herbal medicine is largely due to the Dietary Supplements Health and Education Act (DSHEA) of 1994, which expanded the definition of "dietary supplement" beyond essential nutrients to include "herbs and botanicals," thus removing them from regulation as drugs. This designation means that labels can only mention ways that the herbal product can promote health, not cure disease. For example, valerian root "promotes restful sleep," St. John's wort "may help enhance mood," and Echinacea and goldenseal "may help support the immune system." Table 1 lists some herbal products marketed as food supplements that are currently being tested for efficacy in treating specific illnesses. Many physicians and biochemists argue that active ingredients in many herbal remedies are indeed drugs, and should be regulated as such.
The U.S. Food and Drug Administration does not require food supplements to be tested for safety and efficacy in treating illness, or even that a product be consistent in concentration of the active ingredient, or the plant part from which it is derived. Two-thirds of individuals who take herbal supplements do so without consulting a physician, which can be dangerous. St. John's wort, for example, interacts with enzymes that control blood levels of many drugs, including anesthetics and drugs that transplant recipients must take. Some herbal supplements may be dangerous if taken in large
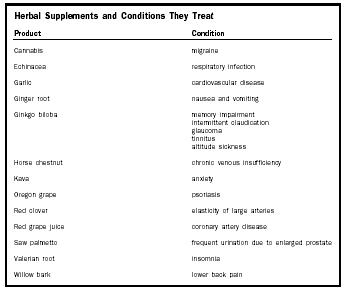
| Herbal Supplements and Conditions They Treat | |
| Product | Condition |
| Cannabis | migraine |
| Echinacea | respiratory infection |
| Garlic | cardiovascular disease |
| Ginger root | nausea and vomiting |
| Ginkgo biloba | memory impairment |
| intermittent claudication | |
| glaucoma | |
| tinnitus | |
| altitude sickness | |
| Horse chestnut | chronic venous insufficiency |
| Kava | anxiety |
| Oregon grape | psoriasis |
| Red clover | elasticity of large arteries |
| Red grape juice | coronary artery disease |
| Saw palmetto | frequent urination due to enlarged prostate |
| Valerian root | insomnia |
| Willow bark | lower back pain |
doses or by individuals with particular illnesses. For example, Ginkgo biloba has been linked to intracranial bleeds, and Ephedra to seizures, hypertension, stroke, and death.
Studies to test effects of herbal substances may be flawed or yield inconsistent results. Some reports are actually studies of studies, selected in a way that prejudices the results. Many trials are too small or not well enough controlled to yield meaningful conclusions. Consider an investigation on whether fruits of the chastetree can prevent symptoms of premenstrual syndrome. For three months, 1,634 women took two capsules a day of the extract, and reported their symptoms before and after the trial period—with no control group not receiving the drug. For St. John's wort, one large investigation found it to be just as effective as a standard antidepressant drug, yet another large study published a few months later found it to be useless.
Not all herbal remedies lack scientific backing due to the peculiarities of regulatory law or variations in experimental design. For example, people have drunk cranberry juice to ease symptoms of urinary tract infections for many years. The effect was thought to be due to increasing acidity of urine, but a 1998 study found that compounds called proanthocyanidins prevent bacterial outgrowths from adhering to the wall of the uterine tract.
It is wise to consult a physician when considering use of an herbal product. Even for a well-understood remedy such as cranberry extract, additional therapy may be required, or drug interactions a possibility. The law may not currently consider herbal ingredients to be drugs, but science indicates otherwise.
Ricki Lewis
Bibliography
Attenborough, David. The Private Life of Plants. Princeton, NJ: Princeton University Press, 1995.
Fleming, G. Alexander. "The FDA, Regulation, and the Risk of Stroke." The New England Journal of Medicine 343, no. 25 (21 December 2000): 1886–1887.
Shelton, Richard C., et al. "Effectiveness of St. John's Wort in Major Depression." The Journal of the American Medical Association 285, no. 15 (18 April 2001): 1978–1986.
Simpson, Beryl Brintnall, and Molly Conner Ogorzaly. Economic Botany: Plants in Our World, 3rd ed. New York: McGraw-Hill Higher Education, 2000.
Comment about this article, ask questions, or add new information about this topic: