Nuclear Transport
The distinguishing feature of eukaryotic cells is the segregation of ribonucleic acid (RNA) synthesis and deoxyribonucleic acid (DNA) replication in the nucleus , keeping it separate from the cytoplasmic machinery for protein synthesis. As a consequence, messenger RNAs, ribosomal RNAs, transfer RNAs, and all cytoplasmic RNAs of nuclear origin must be transported from their site of synthesis in the nucleus to their final cytoplasmic destinations. Conversely, all nuclear proteins must be imported from the cytoplasm into the nucleus.
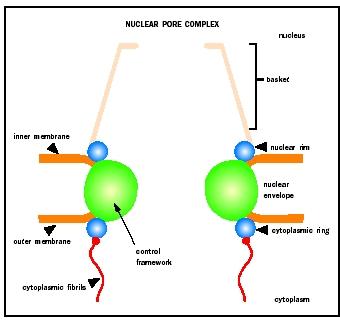
Traffic of macromolecules between the nucleus and cytoplasm occurs through nuclear pore complexes (NPCs). NPCs are large proteinaceous structures that form aqueous channels across the nuclear envelope or membrane. NPCs are composed of multiple copies of up to about fifty proteins termed nucleoporins and consist of three structural units. A ringlike central framework surrounding the central channel of the pore is sandwiched between two peripheral structures: the cytoplasmic ring from which eight cytoplasmic fibrils emanate, and the nuclear rim that anchors the nuclear basket.
Nuclear transport depends on signals for import or export that form part of the transported molecules. These signals are referred to as nuclear localization signals (NLSs) or nuclear export signals (NES), respectively. In proteins, they are specific amino acid sequences. NLSs or NESs are recognized and bound by soluble import or export receptors that shuttle between nucleus and cytoplasm. The interaction of the receptors with their cargoes (or substrates) can be direct or mediated by an additional adapter protein. Upon binding, the transport receptors dock their cargoes to the NPC and facilitate their translocation across the central channel of the pore. After delivering their cargoes, the receptors are recycled to initiate additional
The vast majority of nuclear transport receptors are members of a large family of proteins that exhibit a high affinity for a small GTPase, called Ran, in the GTP bound form. GTP (guanosine triphosphate) is an energy-carrying molecule used in cell signaling. A GTPase like Ran can cause GTP to become GDP (guanosine diphosphate), which will change the properties of the GTPase. The GTPase Ran regulates the interaction of the receptors with their cargoes.
The GTPase acts in concert with several cofactors. The striking property of Ran cofactors is that they are asymmetrically localized in the cell, with some predominantly cytoplasmic while others are predominantly found in the nucleus. This asymmetry helps to control the two-way transport between nucleus and cytoplasm.
SEE ALSO Membrane Transport ; Nucleotides ; Nucleus ; Protein Targeting ; RNA
Elisa Izaurralde
Bibliography
Mattaj, I. W., and L. Englmeier. "Nucleocytoplasmic Transport: The Soluble Phase." Annual Review of Biochemistry (1998) 67: 265-306.
Nakielny, S., and G. Dreyfuss. "Transport of Proteins and RNAs in and Out of the Nucleus." Cell (1999) 99: 677-690.
Comment about this article, ask questions, or add new information about this topic: